Medical Device Manufacturing and Biocompatible Materials
Published on February 24, 2023

Modern medicine relies on a variety of different tools and instruments. Ranging from flexible tubing to gauze to durable metal clamps and prosthetic limbs, these devices take many shapes. Just as the FDA regulates which materials can be used in our food and drug supply chains, they also regulate the development and production of medical devices. This means that certain materials used in industrial applications are not acceptable for use in products that interact with our bodies.
Restrictions around specific materials will vary by region. This means that a device that is approved for use in the United States might not meet the European Union’s standards.
While not every medical device requires biocompatible materials, many do. If the device is intended for internal use it will face stricter scrutiny than devices that might aid in a surgery or are in contact with the skin momentarily. Common examples of medical devices intended for internal use include pacemakers, prosthetics, stents, artificial hips, and other joint replacements.
It’s important that product development teams know which biocompatible materials are best-suited for their specific requirements in order to protect the patient’s health and wellbeing, achieve ongoing compliance with stringent regulations, and mitigate risk and liability. Here are some key guidelines and grounding principles for medical device material selection.
Regulatory Standards for Biocompatible Materials for Medical Devices
The materials and components used by medical device manufacturers must meet the stringent quality and performance requirements of the international regulation ISO 10993, which deals specifically with biocompatibility. ISO 10993 lays out an approach for how to perform risk mitigation and performance testing for device materials in a consistent and uniform manner.
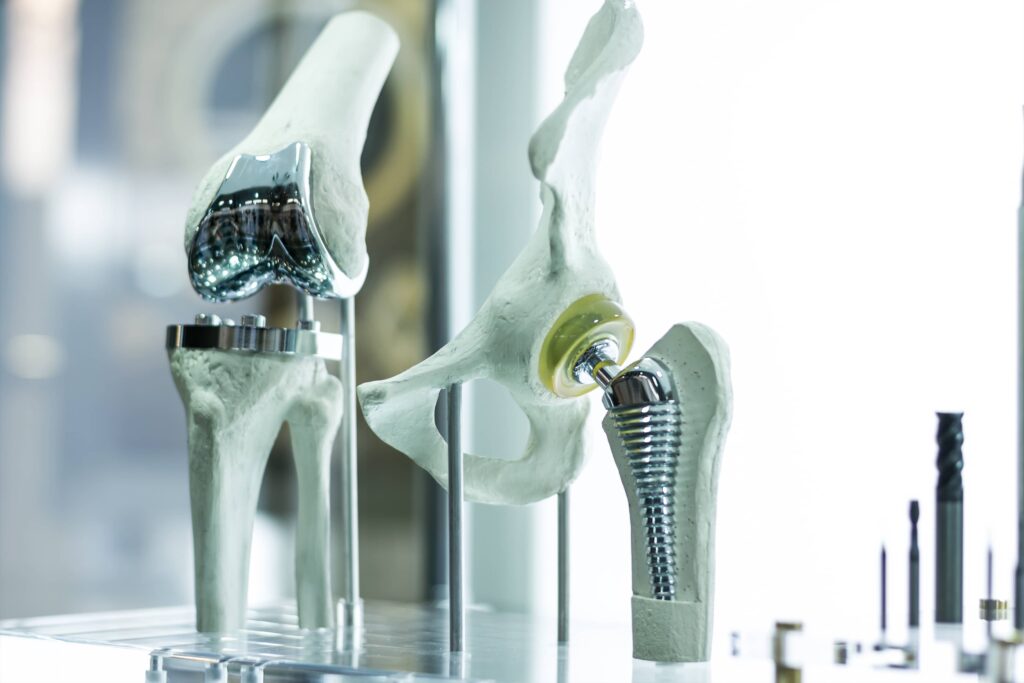
ISO guidelines have the backing of the FDA. In September 2020, the agency released a guidance document offering suggestions for how to implement ISO regulations and ensure that FDA-approved materials for medical devices are in alignment with international standards.
Biocompatibility is a complex and evolving subject with few simple definitions, and the latest update to ISO 10993 guidelines (10993-1:2018; updated from 10993-1:2009) reflects the latest developments in the field. Perhaps the most significant change in the latest edition of ISO 10993 involves how biocompatibility is tested.
Whereas the previous version provided specific tests for assessing the biocompatibility of different device types, the current standard seeks to better address the many variables involved in medical device manufacturing through a comprehensive process of risk assessment, mitigation, and management. This allows the standard to be applied in a wider range of dynamic medical and manufacturing contexts.
The ISO 10993 update also includes additional or updated information about contact and non-contact medical devices, as well methods for evaluating the biocompatibility of nanotechnology, gas pathways, and absorbable materials.
Demonstrating biocompatibility is generally done through a three-stage process:
- Product teams develop a Biological Evaluation Plan (BEP), which outlines known risks and strategies to test or mitigate these concerns. This document fulfills ISO 10993-1’s requirement for an initial risk assessment.
- The device’s materials and components are tested to address these outlined risks, which can include evaluating factors such as how the device wears over time, material toxicity, or how the device operates when it comes in contact with fluids. Often, a variety of test types and design controls for medical devices are necessary to ensure the device functions as intended.
- Product teams consolidate test results and analyses of the data into a Biological Evaluation Report (BER), which they then submit to the FDA for approval.
Additional Biocompatibility Challenges
In addition to achieving compliance with ISO and FDA regulations, biocompatible medical device design can lead to additional challenges for product teams. Medical device product development teams often have specific functional or design-related requirements by which they must adhere, and reconciling these requirements with material restrictions can be a time-consuming and intensive process. In fact, it’s not unheard of for customer requirements to necessitate a contradictory or mutually exclusive set of material properties — and it’s up to product teams to do the research that leads to an acceptable compromise.
Another key challenge involves production timelines. The testing required for toxicology and biocompatibility assessment do not produce simple pass or fail results; rather, these evaluations collectively create a demonstration of compliance or a recommendation for further research and evaluation. Because this requires a thorough and well-documented approach, the certification and approval process for medical devices cannot be rushed. Successful product teams are those with the skill and expertise to meet customers’ requirements while operating in accordance with ISO and FDA regulations.
Key Considerations for Selecting the Right Biocompatible Material
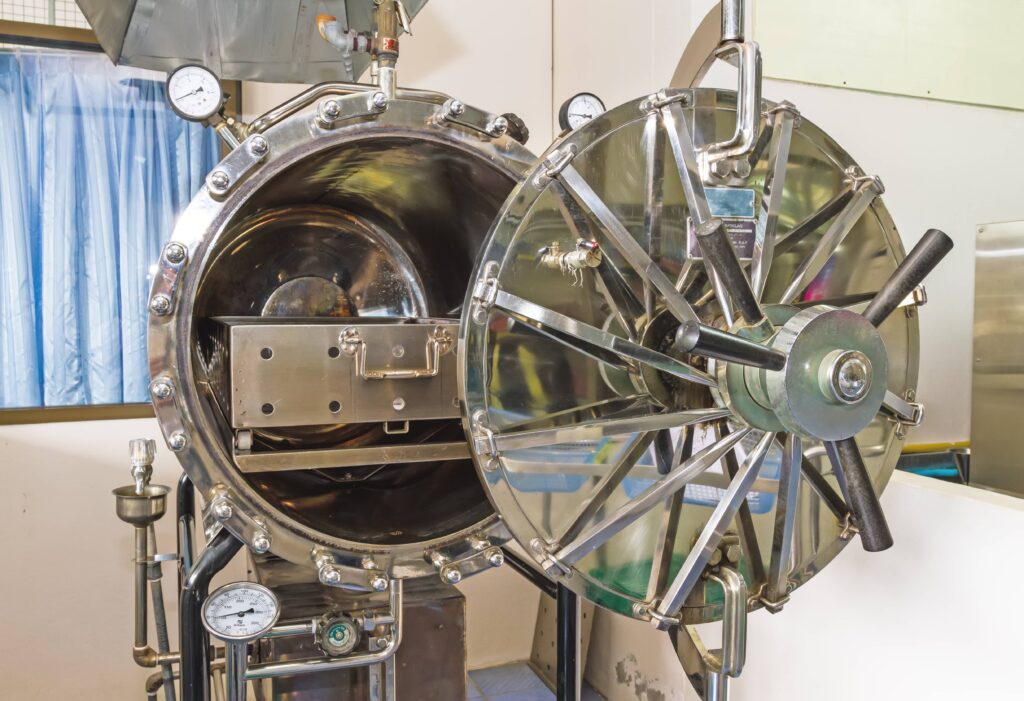
There are numerous variables and factors to take into account when designing and manufacturing biocompatible medical devices, and the specific details will of course vary based on the application.However, choosing the right material is paramount, as researchers have found that 30-40% of device recalls are caused by improper material choice. Here are three key considerations for product teams:
- Material availability: If the design of a medical device includes materials that are scarce or hard to come by, an alternative solution may be necessary. This helps to keep per-unit costs low and to ensure that the device can reach the market on schedule.
- Manufacturing process: The material requirements of a medical device or its components will help determine the optimal production method or methods. Injection molding, for instance, is a rapid and cost-effective means by which to create large quantities of precise plastic components with good surface finishes, but can be extremely expensive for low-volume production. CNC machining, on the other hand, has very few material restrictions but some significant geometric ones. Likewise, developments in additive manufacturing technologies are enabling faster production and greater customization — an especially valuable quality considering the medical sector’s large-scale shift toward patient-centric care — though it’s worth noting that both CNC machining and additive manufacturing are compatible with a comparatively limited range of materials.
- Sterilization needs:Some medical devices and tools, such as hypodermic needles and IV tubing, must be sterilized before they can be circulated back into use. In design terms, this means the device must have a material resistance to the sterilization process. Knowing early on whether a device will have a sterilization requirement — in addition to the sterilization method that will be used — is key to avoiding expensive revisions and tests.
Maintaining an Efficient Design Process During Medical Device Product Development
Given that biocompatibility testing and approval require ongoing evaluation, product development teams will likely need to adapt or rethink their design processes based on their findings.
There are a couple of structural ways in which teams can streamline their design processes. Maintaining an accurate database of materials that includes information related to test results, material toxicology or carcinogenicity, and other characteristics laid out by the ISO 10993, is the first step to creating an archive of historical data that can be referred back to in future design efforts. Doing so not only helps to improve the efficiency of modifications during the design process, but also helps to keep the design team acquainted with the various materials that are relevant to a device’s biocompatibility and functionality requirements.
If component materials have been selected but part geometry has yet to be finalized, plaque testing is a technique that allows teams to stay productive and efficient. This technique involves producing multiple small plaques via the manufacturing method that will be used to create the final product. The plaques are then subjected to biocompatibility testing — including chemical testing and determining how the material breaks down over time — while product developers finalize the part design. This helps to establish the foundation for subsequent evaluation and can speed the regulatory approval process.
Choosing the Right Manufacturing Partner for the Job
The updated processes contained in the latest ISO 10993 seek to minimize unnecessary testing while still guaranteeing that product teams are able to account for how relevant factors like the device design, physical and chemical characteristics of the device materials, and even the manufacturing process can influence the quality of devices and how well they are able to meet patients’ needs. The strenuous design, development, and regulatory processes required for effective medical device manufacturing can present significant challenges for product teams, which is why it’s beneficial to partner with a tried-and-true manufacturer like SyBridge.
SyBridge is an innovative, on-demand digital manufacturing platform with significant experience working with medical device design teams to bring safe, reliable products to market. Our skills and techniques have been used to create cutting edge prosthetics, highly precise surgical models, and more, and our team is prepared to provide 360-degree advisory and support services from the design and prototyping stages to production and fulfillment. Ready to get started? Contact our team today.



