
Modern medicine relies on a variety of different tools and instruments. Ranging from flexible tubing to gauze to durable metal clamps and prosthetic limbs, these devices take many shapes. Just as the FDA regulates which materials can be used in our food and drug supply chains, they also regulate the development and production of medical devices. This means that certain materials used in industrial applications are not acceptable for use in products that interact with our bodies.
Restrictions around specific materials will vary by region. This means that a device that is approved for use in the United States might not meet the European Union’s standards.
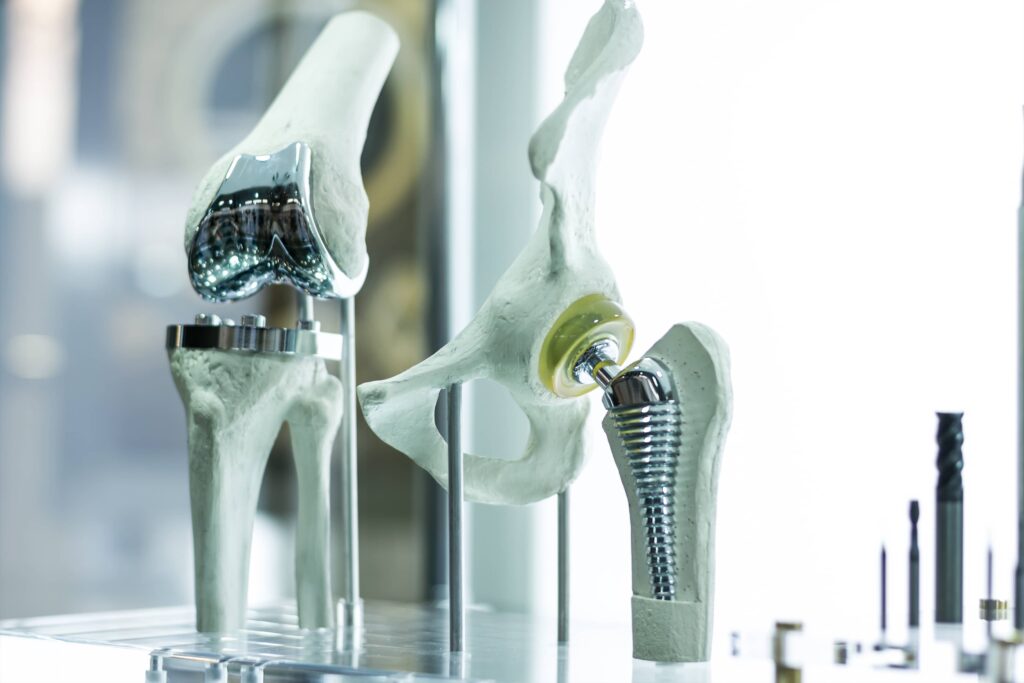
While not every medical device requires biocompatible materials, many do. If the device is intended for internal use it will face stricter scrutiny than devices that might aid in a surgery or are in contact with the skin momentarily. Common examples of medical devices intended for internal use include pacemakers, prosthetics, stents, artificial hips, and other joint replacements.
It’s important that product development teams know which biocompatible materials are best-suited for their specific requirements in order to protect the patient’s health and wellbeing, achieve ongoing compliance with stringent regulations, and mitigate risk and liability. Here are some key guidelines and grounding principles for medical device material selection.
The materials and components used by medical device manufacturers must meet the stringent quality and performance requirements of the international regulation ISO 10993, which deals specifically with biocompatibility. ISO 10993 lays out an approach for how to perform risk mitigation and performance testing for device materials in a consistent and uniform manner.

ISO guidelines have the backing of the FDA. In September 2020, the agency released a guidance document offering suggestions for how to implement ISO regulations and ensure that FDA-approved materials for medical devices are in alignment with international standards.
Biocompatibility is a complex and evolving subject with few simple definitions, and the latest update to ISO 10993 guidelines (10993-1:2018; updated from 10993-1:2009) reflects the latest developments in the field. Perhaps the most significant change in the latest edition of ISO 10993 involves how biocompatibility is tested.
Whereas the previous version provided specific tests for assessing the biocompatibility of different device types, the current standard seeks to better address the many variables involved in medical device manufacturing through a comprehensive process of risk assessment, mitigation, and management. This allows the standard to be applied in a wider range of dynamic medical and manufacturing contexts.
The ISO 10993 update also includes additional or updated information about contact and non-contact medical devices, as well methods for evaluating the biocompatibility of nanotechnology, gas pathways, and absorbable materials.
Demonstrating biocompatibility is generally done through a three-stage process:
In addition to achieving compliance with ISO and FDA regulations, biocompatible medical device design can lead to additional challenges for product teams. Medical device product development teams often have specific functional or design-related requirements by which they must adhere, and reconciling these requirements with material restrictions can be a time-consuming and intensive process. In fact, it’s not unheard of for customer requirements to necessitate a contradictory or mutually exclusive set of material properties — and it’s up to product teams to do the research that leads to an acceptable compromise.
Another key challenge involves production timelines. The testing required for toxicology and biocompatibility assessment do not produce simple pass or fail results; rather, these evaluations collectively create a demonstration of compliance or a recommendation for further research and evaluation. Because this requires a thorough and well-documented approach, the certification and approval process for medical devices cannot be rushed. Successful product teams are those with the skill and expertise to meet customers’ requirements while operating in accordance with ISO and FDA regulations.
There are numerous variables and factors to take into account when designing and manufacturing biocompatible medical devices, and the specific details will of course vary based on the application.However, choosing the right material is paramount, as researchers have found that 30-40% of device recalls are caused by improper material choice. Here are three key considerations for product teams:
Given that biocompatibility testing and approval require ongoing evaluation, product development teams will likely need to adapt or rethink their design processes based on their findings.
There are a couple of structural ways in which teams can streamline their design processes. Maintaining an accurate database of materials that includes information related to test results, material toxicology or carcinogenicity, and other characteristics laid out by the ISO 10993, is the first step to creating an archive of historical data that can be referred back to in future design efforts. Doing so not only helps to improve the efficiency of modifications during the design process, but also helps to keep the design team acquainted with the various materials that are relevant to a device’s biocompatibility and functionality requirements.
If component materials have been selected but part geometry has yet to be finalized, plaque testing is a technique that allows teams to stay productive and efficient. This technique involves producing multiple small plaques via the manufacturing method that will be used to create the final product. The plaques are then subjected to biocompatibility testing — including chemical testing and determining how the material breaks down over time — while product developers finalize the part design. This helps to establish the foundation for subsequent evaluation and can speed the regulatory approval process.
The updated processes contained in the latest ISO 10993 seek to minimize unnecessary testing while still guaranteeing that product teams are able to account for how relevant factors like the device design, physical and chemical characteristics of the device materials, and even the manufacturing process can influence the quality of devices and how well they are able to meet patients’ needs. The strenuous design, development, and regulatory processes required for effective medical device manufacturing can present significant challenges for product teams, which is why it’s beneficial to partner with a tried-and-true manufacturer like SyBridge.
SyBridge is an innovative, on-demand digital manufacturing platform with significant experience working with medical device design teams to bring safe, reliable products to market. Our skills and techniques have been used to create cutting edge prosthetics, highly precise surgical models, and more, and our team is prepared to provide 360-degree advisory and support services from the design and prototyping stages to production and fulfillment. Ready to get started? Contact our team today.
Forget typical cycle times. We're pushing the boundaries of conformal cooling. While traditional approaches deliver…
Forget typical cycle times. We're pushing the boundaries of conformal cooling. While traditional approaches deliver…
From left to right: Brayden Janak (apprentice); Logan Vifaquain (CNC machining, Programming and CMM); Ron…
SyBridge Technologies is proud to announce we have been awarded the 2023 General Motors Supplier…
Today, designers and engineers are accustomed to working with digital tools in their day-to-day jobs.…
Optimizing Your Injection Molding Process for Cost-Effective Manufacturing Excellence In today’s competitive landscape, manufacturers are…